What is hot dip galvanizing
Hot Dip Zinc Plating
Research shows that corrosion is the main responsible for the great loss of iron in the world. Among the protection processes already developed, one of the oldest and most successful is hot-dip galvanizing, or, as it is better known, hot-dip galvanizing.
In 1741, French chemist Melouin discovered that zinc coating could protect steel from corrosion. In 1837, the engineer Sorel patented hot-dip galvanization using the term galvanization (named after Luigi Galvani, 1737-1798, one of the first scientists interested in electricity) because it is the galvanic current that protects the steel. It is called this way because when steel and zinc come into contact in a humid environment, a difference in electrical potential is created between the metals.
Thus, the main objective of Fire Galvanizing is to prevent contact of the base material, steel (Iron Carbon alloy), with the corrosive medium.
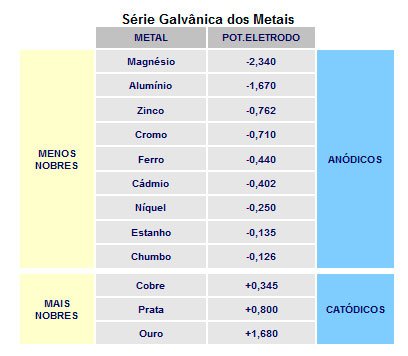
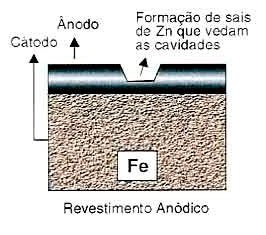
As zinc is more anodic than the iron element in the galvanic series, it is the element that corrodes, creating cathodic protection, that is, the zinc sacrifices itself to protect the iron (see table).
Even if a small area is exposed, the base metal does not suffer the effects of corrosion, as zinc is anodic and will increase its corrosion rate by cathodically protecting the uncovered area.
Process
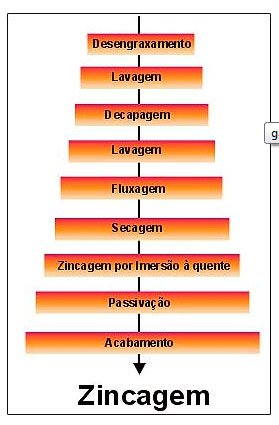
Hot dip zinc plating has a perfectly defined process, being basically the same for any product, and may vary in layer thickness depending on the geometry of the part and the chemical composition of the base material (steel).
Flowchart
SURFACE CLEANING
To obtain a perfect zinc plating finish, the parts must be completely clean, making it necessary to eliminate oils, grease, oxides, glue peels, paints or any other type of substance from the base metal.
To do this, use:
- When degreasing: alkaline degreaser in aqueous solution, hot or cold, to remove organic materials (oils, greases, etc.);
- When pickling: hydrochloric acid, at room temperature, or hot sulfuric acid, to remove iron oxide;
- When fluxing: ZnCl2. 3NH4Cl at a temperature of 60 to 80°C, to dissolve the salt residues remaining on the surface of the piece and form a layer of recrystallization of the salt, which prevents reoxidation in the molten zinc bath;
- When washing: running water with pH control to remove residues between operations, so that one bath does not contaminate the other.
ZINC PLATING
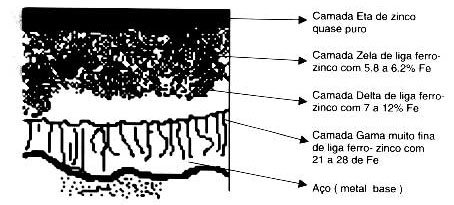
After completing this first stage, the zinc plating phase begins, which consists of immersing the piece in a vat with molten zinc at a temperature between 445 and 460°C, where the iron will react with zinc, starting the formation of four layers. which will form the protective coating.
BASE MATERIAL THAT MAKES UP THE PART
When immersing the part in molten zinc (zinc plating), there are some factors that will influence the formation of the coating.
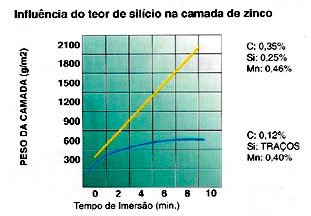
There are some metalloids in the composition of steel that are accelerating elements in the Fe-Zn reaction. Silicon is the greatest activator in the generation of Fe-Zn phases, quickly providing long and thick crystals.
When the content of this element is higher than 0.12%, its effect is already observed with the growth of the Zeta phase to the surface, of the grayish and/or rough coating. The thickness of the coating will be greater than specified, and may be 2x greater.
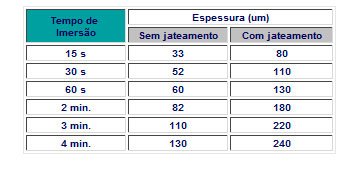
Surface condition: The rougher the surface, the thicker the zinc layer, this is explained by the fact that there is a greater surface area exposed to the reaction between Zn and Fe, providing greater mechanical anchorage of the last layer that is dragged during removal of the part. .
Immersion and removal speed: The immersion must be as quick as possible so that the layer has the same formation time across the entire piece. The recommended speed varies between 6 and 7m/min.
Removal should be slower and more constant to provide a more uniform coating. The last layer (Eta) is formed by dragging material from the surface of the bath during removal. The recommended speed is around 1.5m/min.
Bath temperature: the melting temperature of zinc is around 419°C. The working temperature is between 430 and 460°C.
Higher temperatures accelerate the Fe-Zn reaction, generating coarse and fragile crystallizations with an irregular external appearance, in addition to seriously affecting the useful life of the vat, as above 470°C, the zinc reaction with the vat walls becomes more intense. intense.
Soaking time: The layer grows with immersion time. Up to approximately 1 minute it grows rapidly: from then on it is slow.
The minimum allowable immersion time is that necessary for the entire piece to be at the same temperature as the molten zinc.
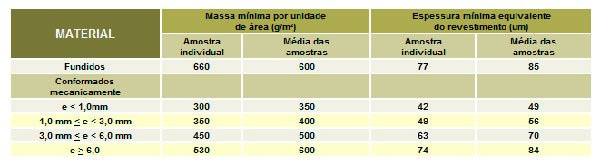
- Galvanizing-Minimum Specified Thickness (one)
- Time for first maintenance in typical locations and adequate zinc thickness. Galvanization – minimum weight specification (g/m²)
Cooling and passivation of the zinc layer: In order for the zinc coating to immediately acquire a protective layer on its surface, passivation is carried out in chromatizing solutions based on chromic acid and bichromate. This passivation gives the zinc-plated product a yellowish appearance.
Finishing
The last stage of this process is the finishing, which can be done through metallization (zinc deposition by thermal spraying) or paint with a high zinc content (greater than 90%).
SOURCE:
Metallic Construction Magazine – Issue no. 50 – ABCEM